Background: Venetoclax (Ven) is a standard of care for the treatment of patients (pts) with CLL/SLL. In the relapsed setting, there is very limited data on the efficacy of Ven after covalent BTK inhibitors ([cBTKi], ibrutinib, acalabrutinib, or zanubrutinib), and there is no prospective trial data on the feasibility of a Ven-based fixed-duration therapy after cBTKi failure. Recently, novel treatment options like pirtobrutinib, a non-covalent BTKi, have shown high efficacy after cBTKi, prompting scrutiny of Ven-based therapies after cBTKi failure, including Ven monotherapy and combination treatment with anti-CD20 antibodies. In this study, we aim to evaluate: 1) The efficacy of Ven-based therapy after cBTKi, 2) Predictors of the efficacy of Ven in this setting, and 3) The feasibility of fixed-duration therapy with Ven after BTKi.
Methods: In this single-institution, retrospective study, we used our institutional CLL registry to identify patients with a CLL/SLL diagnosis who received Ven (with or without anti-CD20 antibodies) for at least two months after prior treatment with a cBTKi. Patient-level clinical data were extracted from electronic medical records. Progressive disease (PD) was defined by the treating physician based on clinical, laboratory, and/or imaging data. Progression-free survival (PFS) and Overall survival (OS) were calculated using the Kaplan-Maier estimates.
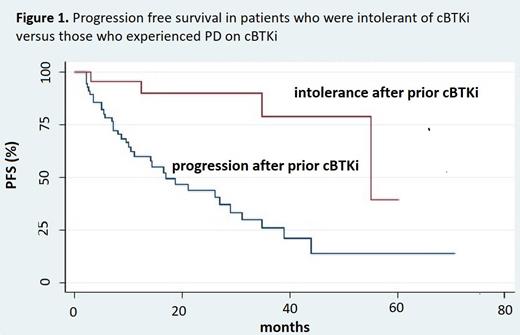
Results: We identified 80 pts who started Ven between 1/2016-12/2022 after prior treatment with a cBTKi. Median age was 62 (range: 40-80), and 53% were male. FISH abnormalities included: 58% del17p, 25% del11q, 48% del 13q, and 19% with trisomy 12. The median prior lines of treatment were 2 (range: 1-7). Prior first cBTKis were ibrutinib (92.5%), acalabrutinib (6.5%), and zanubrutinib (1.5%). A total of 7 pts (9%) received more than one prior cBTKi (1 ibrutinib, 6 acalabrutinib). Prior cBTKi was used as first-line treatment in 21 pts (26%), and 51 (64%) pts received prior treatment with chemotherapy. The median duration of cBTKi treatment was 25 months (range 1-92). Reasons for cBTKi discontinuation were PD (n=56, 70%) and intolerance (n=24, 30%). Most pts started Ven-based therapy immediately after cBTKi (n=53, 66%), although 21 (26%) received one interim line, and 6 (7.5%) received more than one interim line. Ven was used either as monotherapy (n=45, 56%) or with anti-CD20 antibodies (n=35, 44%). The median time on Ven was 11 months (2-55 months). At the time of analysis, 75% of pts had stopped Ven, with the most common reason for discontinuation being PD (31, 53%), followed by treatment completion (19, 32%). Median PFS after starting Ven was 29 months (95% CI: 17-44) for the entire cohort. Median PFS was significantly inferior in pts who experienced PD on prior cBTKi compared with those who were intolerant to prior cBTKi (17 months vs. 55 months, p=0.002). Inferior PFS was also observed in patients with del17p (7 months vs. 26 months without del17p, p=0.003) and in pts who received Ven monotherapy vs. combination therapy with anti-CD20 antibodies (21 months vs. not reached, p=0.01). There was no difference in PFS based on prior treatment with chemotherapy or for pts who received treatments between cBTKi and Ven. In multivariable analysis, having del17p (HR 3.04, 95%CI [1.38-6.65]), Ven monotherapy (HR 3.19, 95% CI [1.52-6.68]) and PD on prior cBTKi (HR 4.29, 95% [1.50-12.29]) were associated with inferior PFS. With a median of 28 months of follow-up, 26 pts (32%) stopped Ven and did not experience PD. This included 23% of pts with PD after prior cBTKi and 54% of pts intolerant to prior cBTKi (p=0.007).
Conclusion: Venetoclax-based therapy after cBTKi provides disease control, but not to the extent reported by the only prospective trial in this setting (Jones, Lancet Oncol, 2018). Inferior PFS was observed in pts who had PD after prior cBTKi (vs. being intolerant), pts who received Ven monotherapy (vs. combined with anti-CD20 antibodies), and those with del17p. While this study may be limited in assessing the feasibility of fixed-duration Ven therapy after cBTKi, our results indicate that such an approach may only be feasible in a subset of pts who stopped cBTKi because of intolerance and not PD.
Disclosures
Lynch:SeaGen: Research Funding; Genentech: Research Funding; Cyteir: Research Funding; Rapt: Research Funding; Cancer Study Group: Consultancy; SeaGen: Consultancy; Foresight Diagnostics: Consultancy; Bayer: Research Funding; Incyte: Research Funding; TG Therapeutics: Research Funding; Abbvie: Consultancy; Merck: Research Funding. Maloney:Gilead Sciences: Consultancy, Honoraria, Other: Member, Scientific Review Committee, Research Scholars Program in Hematologic Malignancies; Incyte: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Participation on a Data Safety Monitory Board , Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Other: Member of the JCAR017 EAP-001 Safety Review Committee and Member, CLL Strategic Council, Member of the JCAR017-BCM-03 Scientific Steering Committee under BMS, Research Funding; Amgen: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Mustang Bio: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: Rights to royalties from Fred Hutch for patents licensed to Juno Therapeutics/BMS, Research Funding; Kite, a Gilead Sciences: Consultancy, Honoraria, Research Funding; A2 Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Other: Member of the Scientific Advisory Board; Legend Biotech: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Other: Chair and Member of the Lymphoma Steering Committee; Navan Technologies: Consultancy, Honoraria, Other: Member of the Scientific Advisory Board; Novartis: Consultancy, Honoraria; Umoja: Consultancy, Honoraria; Bioline Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participation on a Data Safety Monitory Board ; Fred Hutch: Other: rights to royalties for patents licensed to Juno; Navan Technologies: Current holder of stock options in a privately-held company; Chimeric Therapeutics: Other: Member of the Scientific Advisory Board; ImmPACT Bio: Other: Member, Clinical Advisory Board, CD19/CD20 bi-specific CAR-T Cell Therapy Program; Interius: Other: Member, Clinical Advisory Board; Lyell Immunopharma: Other: Member, CAR T Steering Committee. Poh:Incyte: Research Funding; BeiGene: Consultancy; Seattle Genetics: Consultancy; Acrotech: Consultancy. Till:Proteios Technology: Consultancy, Current holder of stock options in a privately-held company; Mustang Bio: Consultancy, Patents & Royalties, Research Funding; BMS/Juno Therapeutics: Research Funding. Smith:ADC Therapeutics, AstraZeneca, BeiGene, Epizyme, Karyopharm, KITE pharma, Incyte, Numab Therapeutics AG, Abbvie, Coherus Biosciences, advisory board (spouse) Genentech, Inc.: Consultancy; BeiGene: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics, AstraZeneca, Ayala (spouse), Bayer, BeiGene, Bristol Myers Squibb (spouse), De Novo Biopharma, Enterome, Genentech, Inc., Ignyta (spouse), Incyte Corporation, Kymera Therapeutics, Merck Sharp and Dohme Corp., MorphoSys, Nanjing Pharmaceu: Research Funding. Gopal:Merck, I-Mab bio, IgM Bio, Takeda, Gilead, Astra-Zeneca, Agios, Janssen, BMS, SeaGen, Teva, Genmab: Research Funding; Compliment Corporation: Current holder of stock options in a privately-held company; Incyte, Kite, Morphosys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, Beigene, Cellectar, Janssen, SeaGen, Epizyme, I-Mab bio, Gilead, Genentech, Lilly, Caribou, Fresenius-Kabi: Consultancy. Ujjani:Genentech: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Lilly: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy; Astrazeneca: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Other: Travel expenses , Research Funding; PCYC: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Atara: Consultancy. Shadman:Mustang Bio: Consultancy, Research Funding; Vincerx: Research Funding; Eli Lilly: Consultancy; Genmab: Consultancy, Research Funding; TG Therapeutics: Research Funding; Janssen: Consultancy; Regeneron: Consultancy; ADC therapeutics: Consultancy; Fate Therapeutics: Consultancy; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; MorphoSys/Incyte: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy; MEI Pharma: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal